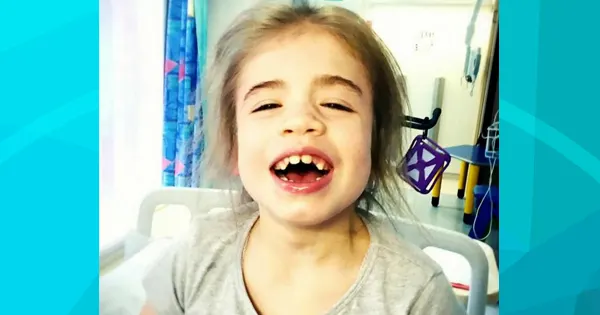
Tillie Mae Mawdsley, nine years old, was diagnosed with Sanfilippo syndrome, which is a degenerative condition also known as similar to childhood dementia. The disorder is caused by the absence of an enzyme that leads to the creation of a build-up composed of sugar around the muscles, which stiffens the joints and causes early death.
According to The Sun, it is extremely rare as it affects only a small number of children. Tillie Mae is slowly degenerating. Michaela, Tillie Mae’s mom, said, “We feel like the rug has been pulled from beneath us and she’s been handed a life sentence. It’s difficult to accept there’s nothing we can do for her now. Tillie’s treatment gave her a quality of life.
“The trial was making her better and I believe that drug was working. It was a huge risk for us to take but I felt like I was doing something. I’m grateful that we were part of it.”
Six years ago, Tillie Mae was fortunate to be included in the six children in the UK offered with the enzyme replacement therapy conducted by Shire, an American pharmaceutical company. Shire stated that no improvement was discovered in the patients’ condition. However, the Mawdsley family were certain that the drug had stopped the symptoms from getting worse.
If a child diagnosed with the Sanfilippo syndrome didn’t get any treatment, they would just continue to regress to the stages of infancy even though they’re growing physically. They will fail to learn and retain even the most basic abilities in life. It will reach to the point that Tillie Mae will lose the ability to breathe.
The oldest expected age a child with the condition can reach is 14 years old.
Michaela, 36 years old, said, “We were grateful that she had the opportunity but she stayed the same and that was enough for us. They wanted to see a change in children and argued the drug wasn’t causing any cognitive improvement.
“They believed it was going to be the next big thing but then one day the trial just stopped and they never really gave us any real reasons why. When Tillie Mae’s sister Lexie found out that the medicine had stopped she said ‘Tillie Mae is going to die now’, it was awful hearing it.
“Being part of something made us feel like we were going somewhere so it’s been a massive blow but we have to be strong for Tillie Mae.”
Part of the causes of the disease is the failure of Tillie Mae to understand what was going on with her memory. Michaela said, “She is doing extremely well but the disease has got her. It’s horrible, it really is like a childhood dementia.”
Michaela and Paul, Tillie Mae’s dad, started a charity in hopes of raising awareness of the degenerative condition and contribute in developing a cure. They have another daughter named Lexie, who’s ten years old.
Michaela said, “We need to raise the funds and raise awareness. We want something here for children suffering in the UK. It would be a dream to find a cure for Sanfilippo. People need to know what it is just like they know what meningitis and Down’s Syndrome is.
“For now, we’re continuing to make memories and just living day by day, taking each day as it comes before Tillie Mae runs out of time. Hopefully in time we can help to find a cure.”
A spokesperson for Shire gave a statement: “Shire is extremely disappointed to have announced that this Phase 2b study did not meet its primary endpoint of slowing the cognitive decline in patients.
“Analysis of secondary endpoints and exploratory endpoints, which were designed to further examine whether there was a therapeutic effect on slowing cognitive decline, an effect on behavioral achievements and slowing the loss of brain volume, were also not met.
“The results did not show any slowing of disease progression in these patients compared to control (untreated) patients. Given the lack of efficacy seen in the results of this study, Shire made the decision to close the MPS IIIA program, which includes discontinuing any ongoing extension studies.
“MPS IIIA is a devastating disease for which there are currently no approved treatment options. We are disappointed for and sympathetic to the Sanfilippo community and thank all the patients and their families for their willingness to participate in these trials.”
source :www.thesun.co.uk